Project J3-3072
Heritable risk for anaphylaxis associated with the increased germline copy number of α-tryptase-encoding sequences at TPSAB1
Research project ARIS
Code: J3-3072
Period 1.10.2021 - 30.9.2024
Head: Assoc Prof Peter Korošec, BSc (Biol), PhD
ARRS classification: Medicine/Human reproduction
Organisations: University Clinic of Respiratory and Allergic Diseases Golnik; University medical centre Ljubljana
Description of the project
Anaphylaxis is a severe, systemic hypersensitivity reaction that is rapid in onset. It is characterized by life-threatening airway, breathing, and/or circulatory problems usually accompanied with skin and mucosal changes. We recently identified (together with the NIH/NSAID), a novel genetic cause for allergic inflammation and immune dysregulation, a common germline genetic trait resulting from increased α-tryptase–encoding sequences at TPSAB1 gene associated with severe anaphylaxis (Lyons, J.J., Chovanec, J., O'Connell, MP,… Korošec, P. 2021, J Allergy Clin Immunol).
This project will contribute to further characterization of this inherited genetic variant, which leads to severe allergic inflammation and anaphylaxis. We will dissect TPSAB1 copy number pathogenesis according to the anaphylactic reactions in children, different triggers of anaphylaxis (food, Hymenoptera venom, and medications), clinical signs and symptoms, potentially near-fatal outcome of anaphylaxis and the natural course of anaphylaxis in adults. Finally, we will functionally characterize blood-derived mast cells from selected anaphylactic subjects with duplication or triplication of α-tryptase–encoding copies at TPSAB1. In this last, functional part of the project, we are hypothesizing that a functional putative mast cell contributing anaphylactic mechanism may exist among individuals with increased α-tryptase–encoding sequences, reflecting a gene dose-dependent mast cell hyperresponsiveness. A role for tryptases in anaphylaxis is relevant to recent efforts toward developing a targeted mAb that neutralizes tryptase proteolytic activity and limits anaphylaxis severity in a humanized mouse model. Our data might suggest that patient outcomes in clinical trials targeting tryptases for neutralization or inhibition may be significantly affected by tryptase genetic variation and that tryptase genotyping may help identify individuals in whom these personalized therapies may be of most benefit.
Project timeline
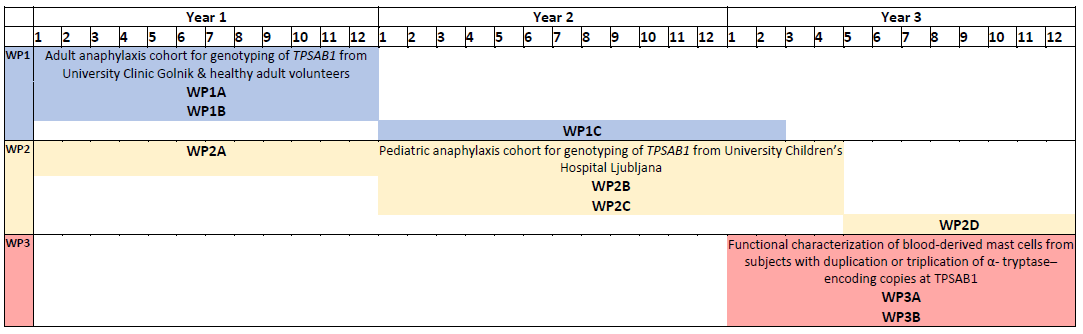
Project work packages and their realization
|
Work package (WP) |
Tasks |
Milestones &Deliverables |
Work distribution |
|
WP1 Adult anaphylaxis cohort for genotyping of TPSAB1 from University Clinic Golnik & healthy adult volunteers |
WP1A Clinical characterization of cohort: different triggers of anaphylaxis, clinical signs and symptoms, the near-fatal outcome of anaphylaxis, and natural course |
Detailed cohort data |
University Clinic Golnik
|
|
WP1B Genotyping of TPSAB1 (β/β, α/β, α/α, αα/β, αα/α, αα/αα, ααα/β and ααα/α) |
TPSAB1 genotypes |
||
|
WP1C Analysis, publications, clinical implementations |
Manuscripts, guidelines |
||
|
WP2 Pediatric anaphylaxis cohort for genotyping of TPSAB1 from University Children’s Hospital Ljubljana
|
WP2A Recruitment of children with anaphylaxis
|
Recruited pediatric cohort |
University Children’s Hospital Ljubljana
|
|
WP2B Clinical characterization of cohort: different triggers of anaphylaxis, clinical signs and symptoms, the near-fatal outcome of anaphylaxis, and natural course
|
Detailed cohort data |
||
|
WP2C Genotyping of TPSAB1 (β/β, α/β, α/α, αα/β, αα/α, αα/αα, ααα/β and ααα/α) |
TPSAB1 genotypes |
University Clinic Golnik |
|
|
WP2D Analysis, publications, clinical implementations |
Manuscripts, guidelines |
University Children’s Hospital Ljubljana |
|
|
WP3 Functional characterization of blood-derived mast cells from subjects with duplication or triplication of α-tryptase–encoding copies at TPSAB1 |
WP3A Generation of primary mast cells from peripheral blood from subjects with selected duplication or triplication of α-tryptase–encoding copies at TPSAB1 & healthy adult volunteers |
Mast cells from peripheral blood precursors
|
University Clinic Golnik
|
|
WP3B Functional characterization utilizing passive sensitization, expressions of CD63 activation marker and secretion of CCL2, PGD2, β-hexosaminidase, and heterotetrameric α/β -tryptase |
Confirming or rejecting hyperresponsiveness hypothesis, Manuscripts |
The project is being implemented in accordance with the set goals and timelines.
Bibliographic references that come directly from the implementation of the project:
ŠELB, Julij, RIJAVEC, Matija, ERŽEN, Renato, ZIDARN, Mihaela, KOPAČ, Peter, ŠKERGET, Matevž, BAJROVIĆ, Nisera, DEMŠAR LUZAR, Ajda, PARK, Young Hwan, LIU, Yihui, ČURIN-ŠERBEC, Vladka, ZVER, Samo, KOŠNIK, Mitja, LYONS, Jonathan J., KOROŠEC, Peter. Routine KIT p.D816V screening identifies clonal mast cell disease in Hymenoptera allergic patients regularly missed using baseline tryptase levels alone. The journal of allergy and clinical immunology. [Online ed.]. Aug. 2021, vol. 148, iss. 2, str. 621-626.e7, ilustr. ISSN 1097-6825. https://www.jacionline.org/action/showPdf?pii=S0091-6749%2821%2900423-1, DOI: 10.1016/j.jaci.2021.02.043. [COBISS.SI-ID 56665603], [JCR, SNIP, WoS do 26. 10. 2022: št. citatov (TC): 6, čistih citatov (CI): 4, čistih citatov na avtorja (CIAu): 0,34, Scopus do 15. 10. 2022: št. citatov (TC): 10, čistih citatov (CI): 8, čistih citatov na avtorja (CIAu): 0,68]
kategorija: 1A1 (Z, A'', A', A1/2); uvrstitev: SCIE, Scopus, MBP (BIOABS, BIOPREW, GEOREF, IPA, CAB, FSTA, MEDLINE, PUBMED); tip dela je verificiral OSICM
točke: 14.12, št. avtorjev: 1
ŠELB, Julij, RIJAVEC, Matija, KOPAČ, Peter, LYONS, Jonathan J., KOROŠEC, Peter (avtor, korespondenčni avtor). Hα" role="presentation" id="MathJax-Element-3-Frame">αT is associated with increased risk for severe Hymenoptera venom–triggered anaphylaxis. The journal of allergy and clinical immunology. [Online ed.]. 2022, vol. 150, iss. , str. 1-2, tabela. ISSN 1097-6825. https://www.jacionline.org/action/showPdf?pii=S0091-6749%2822%2901623-2, DOI: 10.1016/j.jaci.2022.11.017. [COBISS.SI-ID 135585283], [JCR, SNIP]
kategorija: 1A1 (Z, A'', A', A1/2); uvrstitev: SCIE, Scopus, MBP (BIOABS, BIOPREW, GEOREF, IPA, CAB, FSTA, MEDLINE, PUBMED); tip dela še ni verificiran
točke: 33.22, št. avtorjev: 5
CHOVANEC, Jack, TUNC, Ilker, HUGHES, Jason, HALSTEAD, Joseph, MATEJA, Allyson, LIU, Yihui, ŠELB, Julij, RIJAVEC, Matija, KOROŠEC, Peter, LYONS, Jonathan J. (avtor, korespondenčni avtor), et al. Genetically determining individualized clinical reference ranges for the biomarker tryptase can limit unnecessary procedures and unmask myeloid neoplasms. Blood advances. 2022, vol. 6, iss. , str. 1-41, ilustr. ISSN 2473-9529. https://ashpublications.org/bloodadvances/article-pdf/doi/10.1182/bloodadvances.2022007936/1923398/bloodadvances.2022007936.pdf, DOI: 10.1101/2022.04.29.22274379. [COBISS.SI-ID 125092355], [JCR, SNIP]
kategorija: 1A1 (Z, A', A1/2); uvrstitev: SCIE, Scopus, MBP (BIOABS, BIOPREW, MEDLINE, PUBMED); tip dela še ni verificiran
točke: 6.76, št. avtorjev: 45
RIJAVEC, Matija (avtor, korespondenčni avtor), MAVER, Aleš, TURNER, Paul J., HOČEVAR, Keli, KOŠNIK, Mitja, YAMANI, Amnah, HOGAN, Simon P., CUSTOVIC, Adnan, PETERLIN, Borut, KOROŠEC, Peter. Integrative transcriptomic analysis in human and mouse model of anaphylaxis identifies gene signatures associated with cell movement, migration and neuroinflammatory signalling. Frontiers in immunology. 2022, vol. 13, str. 1-15, ilustr. ISSN 1664-3224. https://www.frontiersin.org/articles/10.3389/fimmu.2022.1016165/pdf, DOI: 10.3389/fimmu.2022.1016165. [COBISS.SI-ID 133240067], [JCR, SNIP, WoS]
kategorija: 1A1 (Z, A'', A', A1/2); uvrstitev: SCIE, Scopus, MBP (CAB, MEDLINE, PUBMED, DOAJ); tip dela je verificiral OSICB
točke: 11.29, št. avtorjev: 10
KOPAČ, Peter, CUSTOVIC, Adnan, ZIDARN, Mihaela, ŠILAR, Mira, ŠELB, Julij, BAJROVIĆ, Nisera, ERŽEN, Renato, KOŠNIK, Mitja, KOROŠEC, Peter. Biomarkers of the severity of honeybee sting reactions and the severity and threshold of systemic adverse events during immunotherapy. The journal of allergy and clinical immunology. In practice. [Online ed.]. Aug. 2021, vol. 9, no. 8, str. 3157-3163.e5, ilustr. ISSN 2213-2201. https://www.jaci-inpractice.org/article/S2213-2198(21)00507-9/abstract, DOI: 10.1016/j.jaip.2021.04.045. [COBISS.SI-ID 62025475], [JCR, SNIP, WoS do 26. 10. 2022: št. citatov (TC): 5, čistih citatov (CI): 4, čistih citatov na avtorja (CIAu): 0,44, Scopus do 11. 1. 2023: št. citatov (TC): 7, čistih citatov (CI): 6, čistih citatov na avtorja (CIAu): 0,67]
kategorija: 1A1 (Z, A'', A', A1/2); uvrstitev: SCIE, Scopus, MBP (MEDLINE, PUBMED); tip dela je verificiral OSICM
točke: 15.1, št. avtorjev: 9


 04 25 69 100
04 25 69 100